PharmaSuite v12.00.00 Press Release: Unlocking faster, scalable, and more secure deployments for pharmaceutical and biopharmaceutical manufactures.
FactoryTalk®PharmaSuite® is the leading Manufacturing Execution System (MES) solution purpose-built for pharmaceutical and biopharmaceutical manufacturing. By integrating seamlessly, from enterprise systems to the shop floor, PharmaSuite delivers the precision, control, and reliability that leading manufacturers demand in today’s increasingly complex production environment.
Why PharmaSuite®?
PharmaSuite provides role-based optimization for each lifecycle stage and drives time to results for all users. Its open-content architecture, paired with an intelligent upgrade engine, enables a robust system for growth in both batch and discrete processing.
Scalable applications include:
- Production order management
- Quality management
- Recipe management
- Review by exception
- Track and trace
What’s NEW with PharmaSuite 12.00.00
Cloud-based centralized system deployment
Efficient multi-version upgrade
New out-of-the-box functionality
Minimize manual efforts and reduce downtime
Central Infrastructure & Application Monitoring
Containerized solutions deployed into kubernetes environment
Continued Cyber Monitoring and Hardening
Laying the foundation for growth
As pharmaceutical manufacturers face unprecedented growth and regulatory complexity, the imperative for robust manufacturing execution systems has never been more critical. See how Life Sciences companies are navigating the transition from traditional paper-based processes to digital solutions. Learn why leading pharmaceutical companies are reimagining their production systems to meet escalating demand while maintaining stringent quality standards in an increasingly competitive global market.
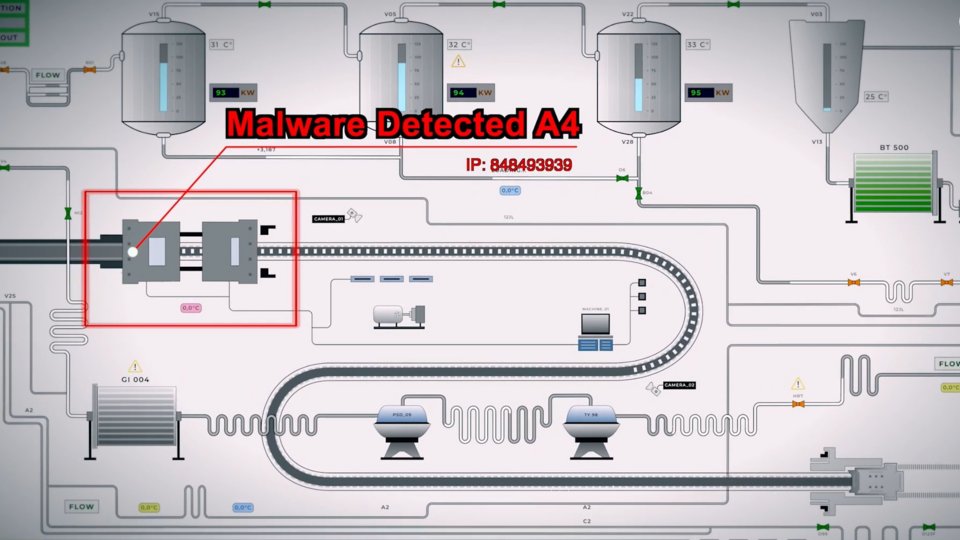
Mitigate risk with network solutions for pharma
Cybersecurity risk has steadily increased in the Life Sciences sector. As process gain complexity and require highly digital environments for fast, seamless data transfer, facilities are more exposed than ever.
Learn how implementing a network of solutions can help monitor activity around the clock and provide end-to-end protection.

Pharma’s Digital Foundation
When Rottendorf Pharma set out to digitally transform their pharmaceutical manufacturing operations, they knew the foundation had to be rock solid. This German CDMO had built its reputation on excellence in solid-dose production- but excellence wasn’t enough. They needed a digital infrastructure that could scale with their ambitions and keep up with market demand. Discover how Rottendorf Pharma’s strategic approach to digital transformation not only streamlined their operations but created a blueprint that’s catching the attention of pharmaceutical manufacturers worldwide.
Webinar
Cold Chain Tracking with PharmaSuite
Manage and track thermo-sensitive material throughout the entire production process. Define temperature and time while setting operator notifications for improved quality and reduce wasted product.
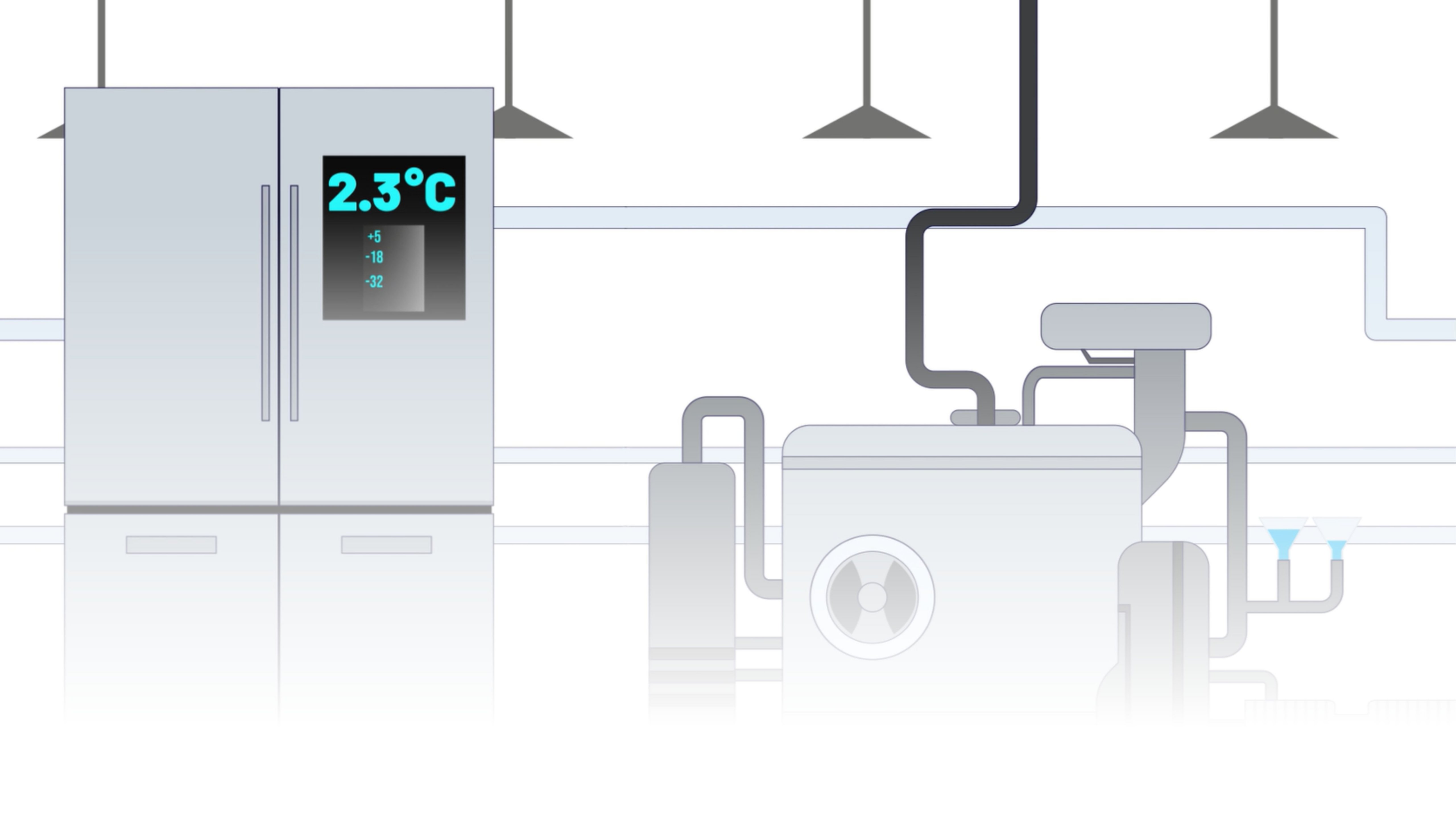
Preserve Quality with Cold Chain Tracking
With PharmaSuite, manufacturers can manage and track thermo-sensitive material throughout the entire production process to:
- Define temperature and time requirements for all thermo-sensitive materials being used
- Track the amount of time a sublot has spent in each temperature range
- Notify operators when a sublot is reaching usable limits
- Manage cold chain tracking properties inheritance

Ready to see more? Click below to schedule an in-depth demo.

Got questions? Click below to connect with one of our smart manufacturing experts.

Get the latest in smart manufacturing technology and fuel your digital transformation.
Recommended for You
You may also be interested in