Downloads
What is it for?
21 CFR Part 11 is a regulation issued by the U.S. Food and Drug Administration (FDA) in 1997. It establishes the criteria under which electronic signatures and records are accepted as legal equivalents to handwritten signatures and paper records in the life sciences industry (pharmaceutical, biotechnology, medical device, etc.).
Its objective is to ensure that computer systems used in manufacturing, quality control, and regulatory documentation meet standards of:
- Authenticity
- Integrity
- Confidentiality
- Non-repudiation (a signature cannot be rejected as non-genuine)
Implementing 21 CFR is useful because it:
- Replaces paper: Reduces storage, printing, and physical handling costs.
- Streamlines processes: Enables legally valid digital workflows.
- Efficient auditing: Facilitates tracking of actions and changes in electronic records.
- Regulatory compliance: This is mandatory for companies operating under the FDA.
General Features
- Closed vs. open systems: Define controls based on who manages access.
- Electronic records: Text, graphics, audio, data, etc., in digital format.
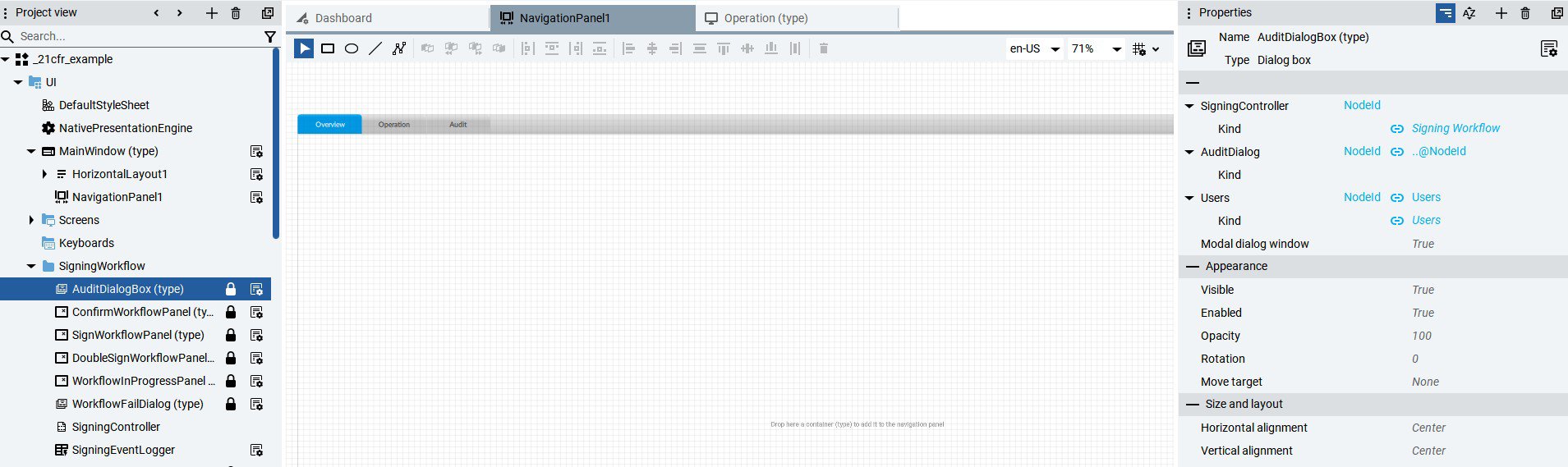
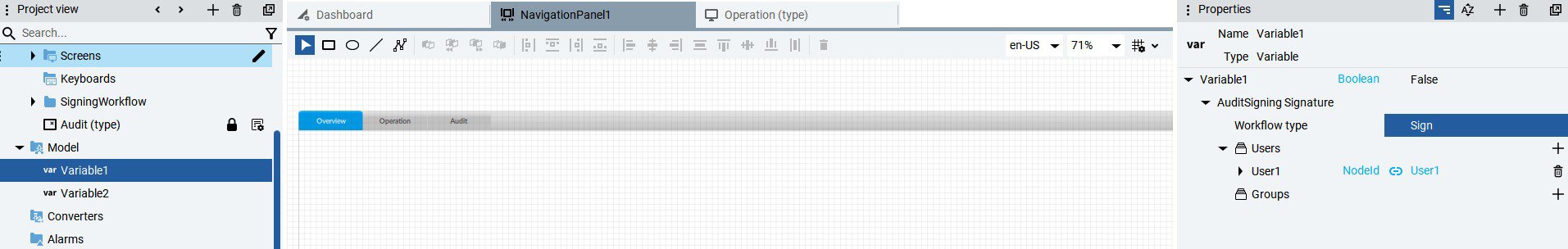
- Electronic signatures: Legal equivalents of handwritten signatures.
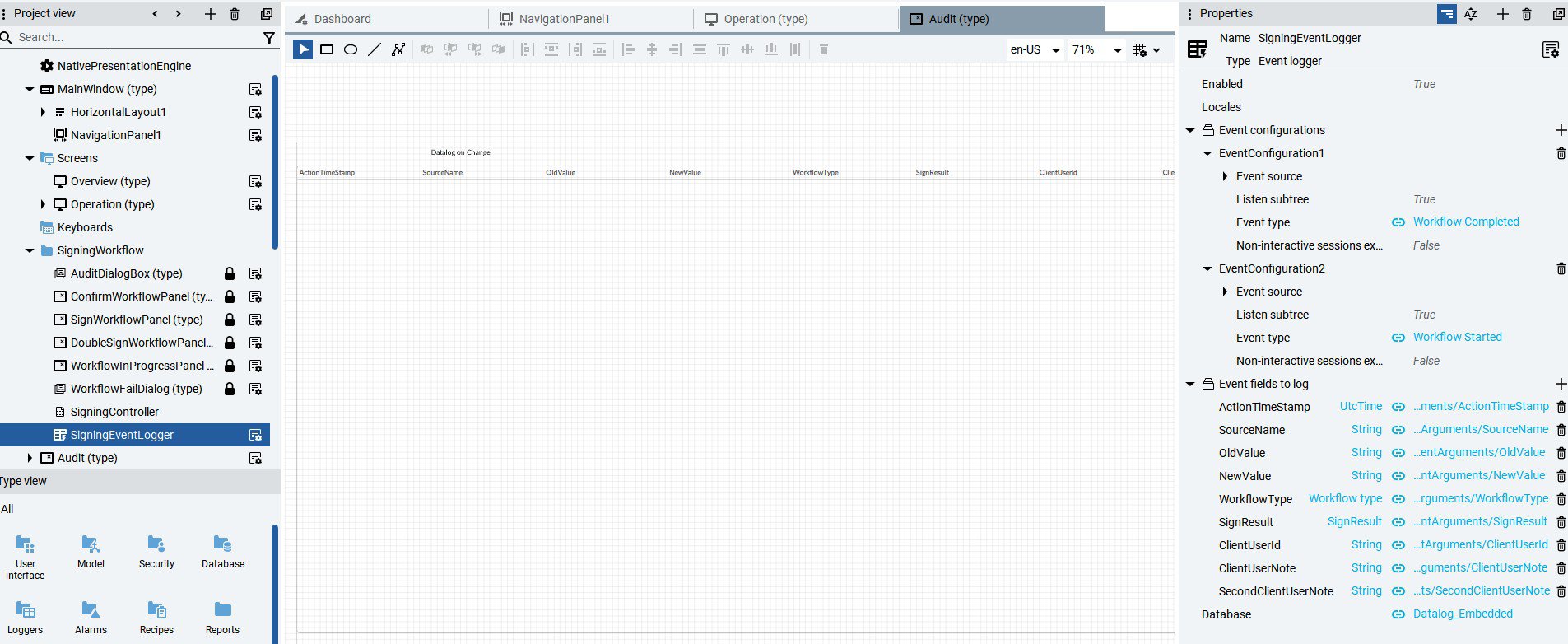
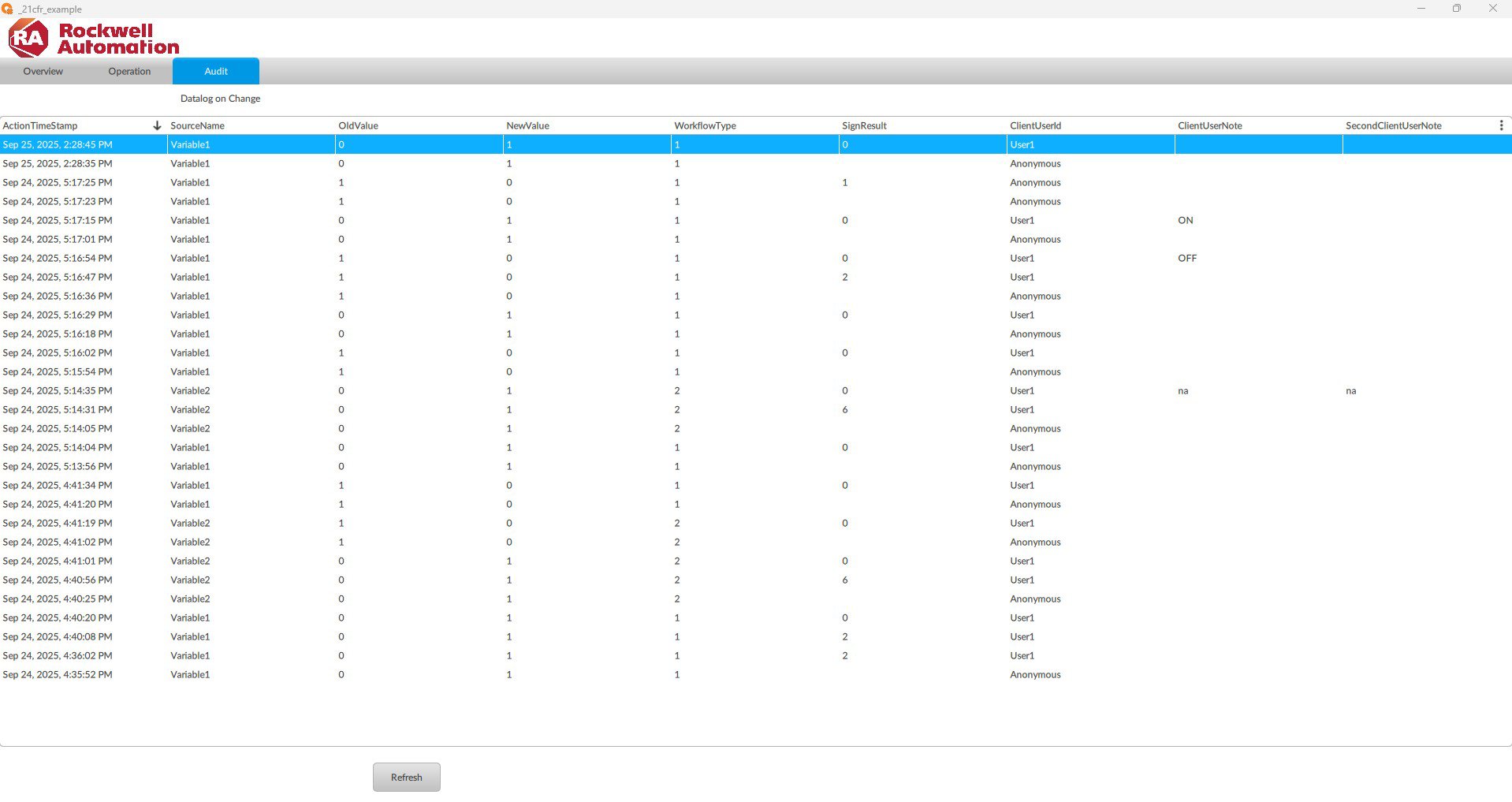
- Automated audits: Records of who modified what, when, and what.
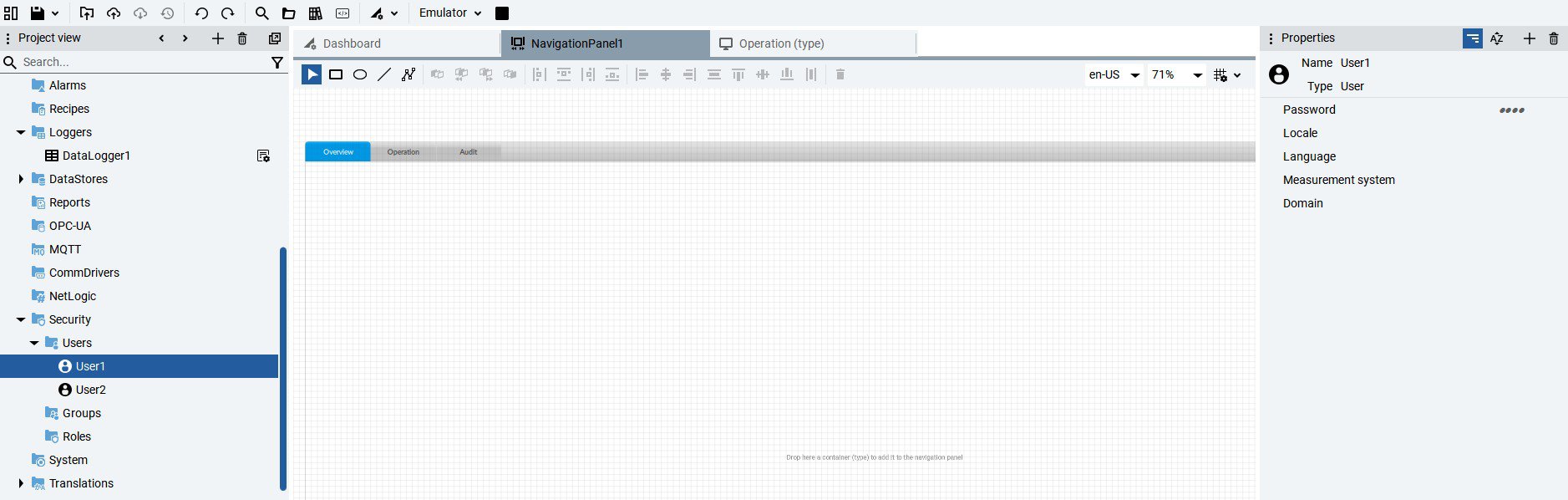
- Access controls: User authentication and role-based permissions.
Advantages:
- Complete traceability: Every action is logged with a username and timestamp.
- Security: Access controls, encryption, and password policies.
- Efficiency: Digital approval workflows with electronic signatures.
- Flexibility: Configurable according to each company's specific needs.
Limitations/Disadvantages
- Implementation complexity: Requires detailed security configuration and auditing.
- Initial costs: Investment in compatible software and training.
- Maintenance: Updates, backups, and user management.
- Rigidity: Changes must follow validated procedures.
Need Help?
If you need help with an application or have feedback from the Innovation Center, please contact us.
Is this useful for me?
This code is defined for applications where you need to implement 21CFR.
Application areas: Food, Manufacturing, Beverage
How can I make it work?
- Hardware: Personal computer
- Software: FT Optix
- Knowledge: Knowledge of programming and configuration in FT Optix and application of the 21CFR standard.
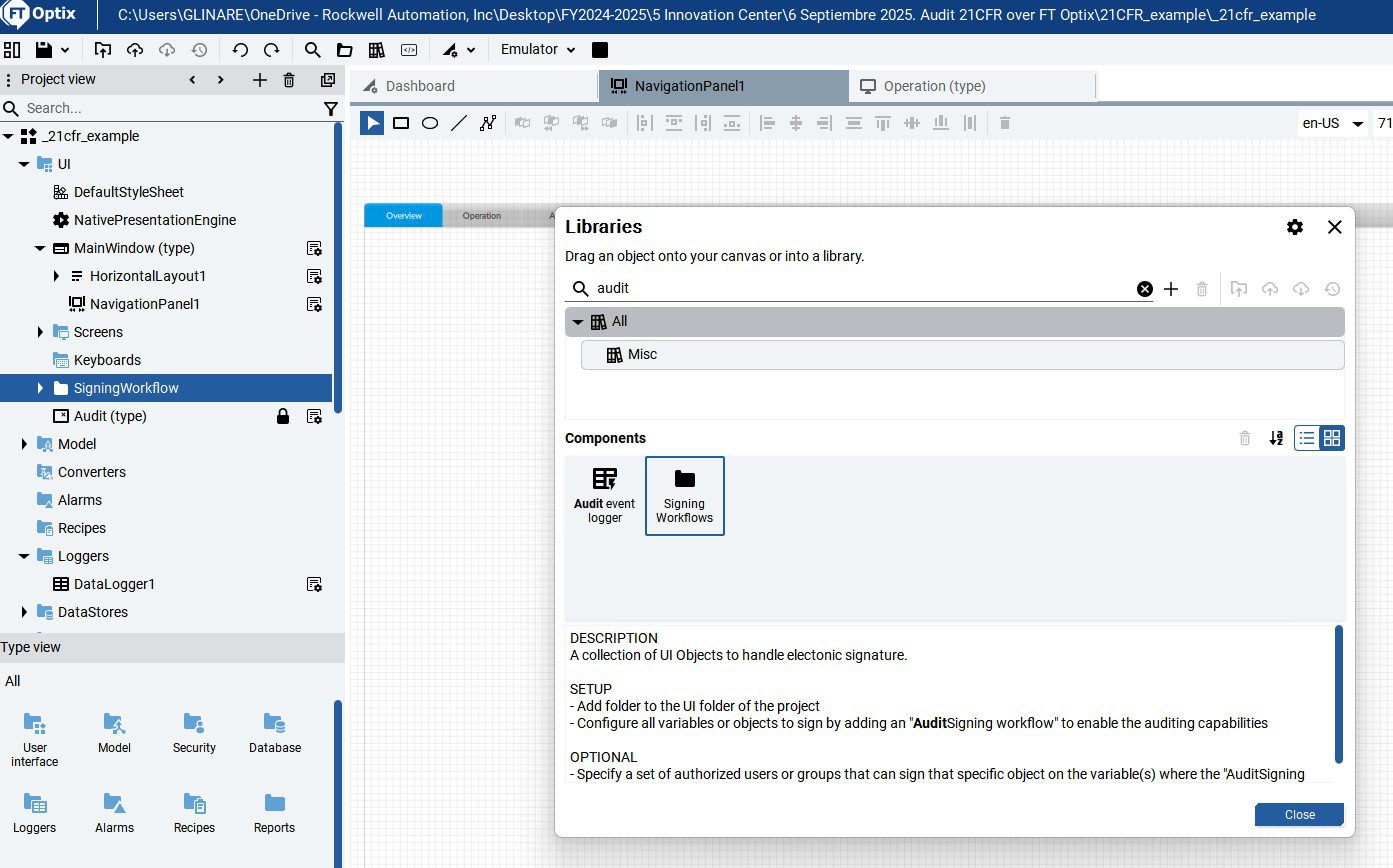
Installation guide
To implement, please follow the steps below.

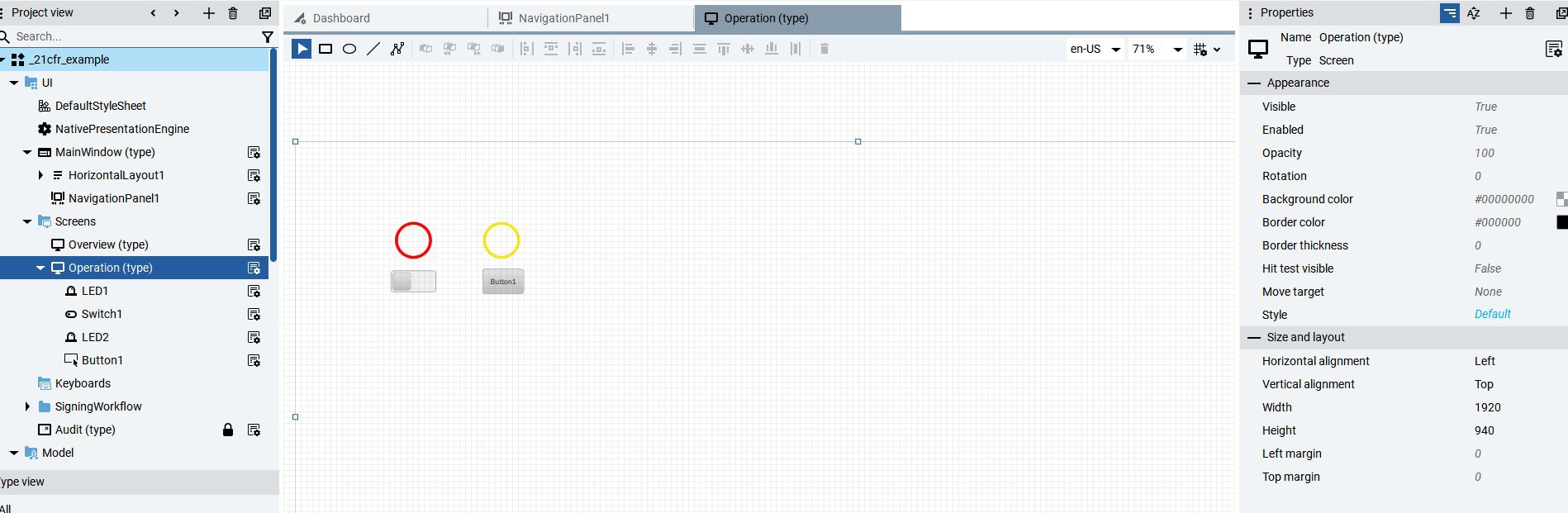
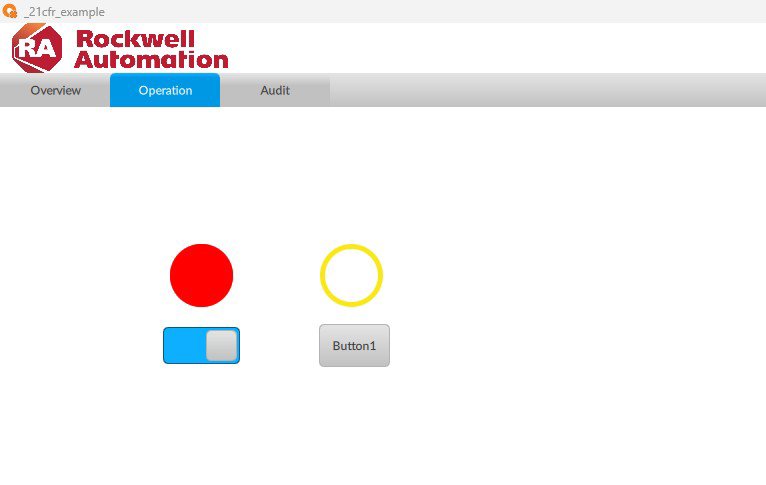
Implement AUDIT TRAIL 21CFR in FT Optix
Version 1.0 - September 2025